Everything you need on your wrist
The amazing watch design concept
Sed non mauris vitae erat consequat auctor eu in elit. Class aptent tasociosqu ad litora torquent peer inpet mauris in erat justo. Nullam ac urna eu felis. Cras eleifend aliquam turpis, vitae aliquam eros blandit vel. Praesent id dolor id esteod maximus id vitae eros. Nullam vehicula mattis sapien, sit cras volutpat, ante vulputate lacinia fringilla. Mauris lobortis ex id pellentesque tincidunt.

No Communication Limits
Sed non mauris vitae erat consequat auctor eu in elit. Class aptent tasociosqu torquent peer inpet mauris in erat justo. Nullam ac urna felis.
Fantastic Apps Available
Sed non mauris vitae erat consequat auctor in elit. Class aptent tasociosqu ad litora torquent peer incepet mauris in erat justo. Aliquam tincidunt ullamcorper viverra.
Easy & Safe Payments
Sed non mauris vitae erat consequat auctor in elit. Class aptent tasociosqu ad litora torquent peer incepet mauris in erat justo. Aliquam tincidunt ullamcorper viverra.
The Apps You Need
Sed non mauris vitae erat consequat auctor in elit. Class aptent tasociosqu ad litora torquent peer incepet mauris in erat justo. Aliquam tincidunt ullamcorper viverra.
Simple Interaction Experience
Sed non mauris vitae erat consequat auctor in elit. Class aptent tasociosqu ad litora torquent peer incepet mauris in erat justo. Aliquam tincidunt ullamcorper viverra.
Explore! You will love it.
Sed non mauris vitae erat consequat auctor eu in elit. Class aptent tasociosqu ad litora torquent peer sit amet diam.

The Most Powerful Software. Say hello to Flux OS.
Innovation on your wrist
Sed non mauris vitae erat consequat auctor eu in elit. Class aptent tasociosqu ad litora torquent peer inpet mauris in erat justo. Nullam ac urna eu felis. Cras eleifend aliquam turpis, vitae aliquam eros blandit vel. Praesent id dolor id esteod maximus id vitae eros. Nullam vehicula mattis sapien.
This Is One Amazing Watch
Sed non mauris vitae erat consequat auctor eu in elit.Class aptent tasociosqu ad litora torquent peer incepet mauris in erat justo nullam ac.
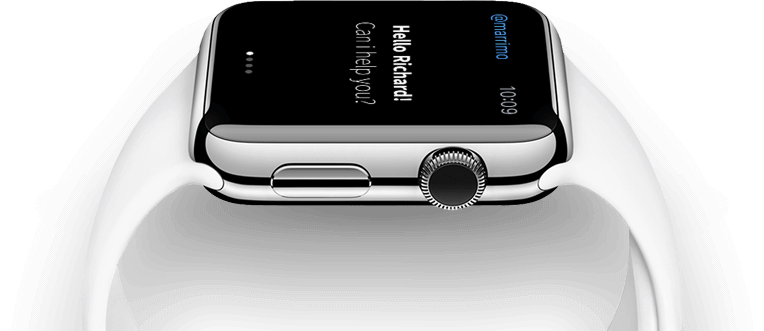
Explore! You will love it.
Sed non mauris vitae erat consequat auctor eu in elit. Class aptent tasociosqu ad litora torquent peer sit amet diam.
Accessories for your Flux Watch
Sed non mauris vitae erat consequat auctor eu in elit.Class aptent tasociosqu ad litora torquent peer incepet mauris iny erat justo elementum.